The science background section gives teachers more in-depth information on the phenomena students explore in this unit. Below is an excerpt from the science background on natural resources.
Fossils and Fuel
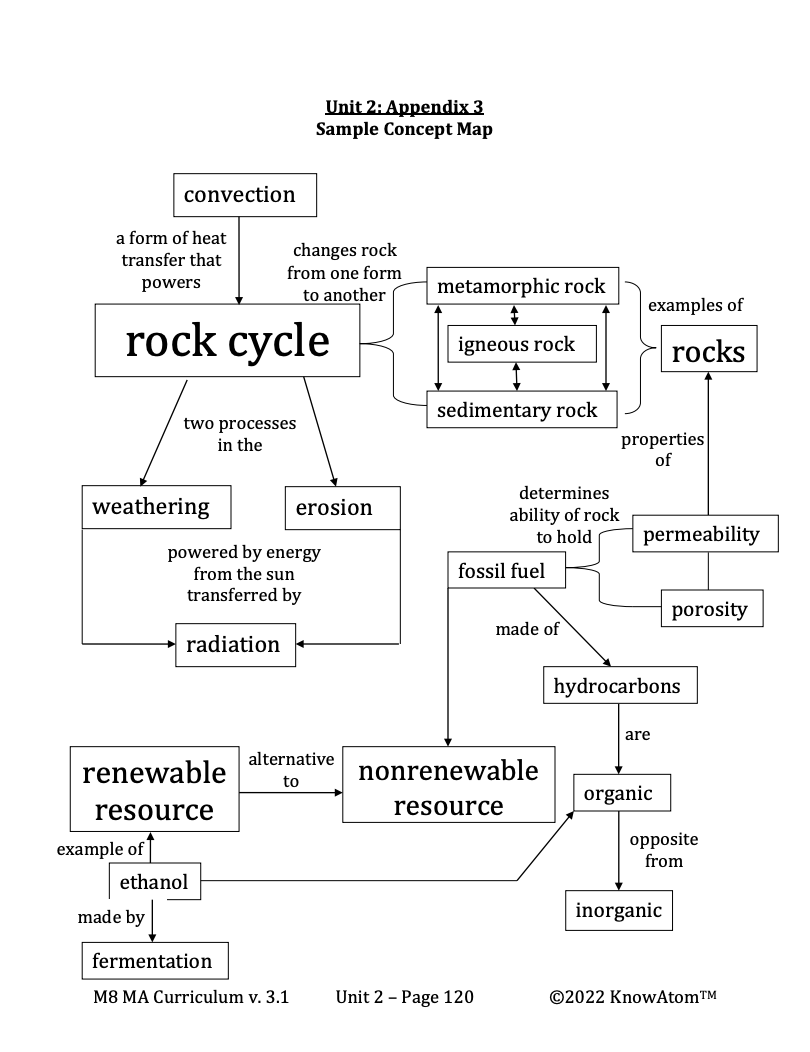
Scientists can read the size, shape, and arrangement of sedimentary particles to get an idea of the environmental conditions on Earth over time. The type of sediment and fossils found in sedimentary rock may reveal ancient oceans, glaciers, and swamps that existed and disappeared over time. Sedimentary rock can also tell geologists something very valuable: that crude oil or natural gas may be present.
Long before the dinosaurs roamed, more than 300 million years ago, oceans covered most of Earth’s surface. The oceans teemed with tiny plants and animals called plankton. After these organisms died, they sank to the bottom of the ocean. Over time, sand and mud covered their remains.
As the once-living remains decomposed, they formed organic material that mixed with the sediment. Organic material is anything that is living or was once living, such as fallen leaves or animal remains. Anything that is not living and never was living is inorganic. Water and rocks are both inorganic. As new sedimentary layers were deposited, the layers put tremendous pressure on the sediment and organic matter, while Earth’s interior provided heat.
The heat and pressure turned the organic material into coal, crude oil, and natural gas. This is why coal, oil, and natural gas are called fossil fuels—they are nonrenewable energy sources that formed millions of years ago from plant and animal matter.
Chemical Reactions
The chemical reactions that transform dead organic matter into new substances with different properties require energy. That energy comes from deep within Earth’s interior. Just like matter, energy is never created or destroyed. This means that there is the same amount of energy today as there was during the time of the dinosaurs, but energy is always on the move, transforming from one form to another.
Remember that all chemical reactions require energy because it takes energy to break the chemical bonds holding atoms and molecules together, and it takes energy to form new bonds. In exothermic reactions, more energy is needed to break the bonds of the reactants than is needed to form the new bonds of the products. As a result, energy is released into the environment. In endothermic reactions, more energy is needed to form the bonds of the products than is needed to break the bonds of the reactants. That is why endothermic reactions absorb energy from their surroundings, becoming cooler.
Photosynthesis is an example of an endothermic reaction that is fundamental to all life. During photosynthesis, plants convert energy from the sun into a usable form of energy. The absorbed energy allows an endothermic reaction involving water and carbon dioxide to take place. Plants and animals store that energy, and it remains stored for millions of years as the organic remains turn into fossil fuels.
That stored energy is released in an exothermic reaction when the fossil fuels are burned. Exothermic reactions release thermal energy, making the surrounding temperature warmer. Exothermic chemical reactions that burn fossil fuels are some of the primary sources of energy in the United States.
Looking for Oil
Oil companies spend vast amounts of time, effort, and money searching for fossil fuels. Once geologists have a good sense of how fossil fuels are formed, they then look for clues in Earth’s geology to try to figure out where fossil fuels might be found. It’s not easy, because fossil fuels rarely stay where they are formed. Because Earth is a dynamic planet, once oil and gas are formed within rocks, they continue to move around the planet, in ways both big and small.
Let’s start with the small movements. Oil and gas form into a type of sedimentary rock called source rock—rock from which hydrocarbons are formed. Source rocks have organic material from the once-living organisms, as well as inorganic sediment.
Over time, crude oil flowed from the source rock and began to accumulate in a reservoir rock. Reservoir rocks hold oil and gas like a sponge. This is because they tend to have a lower density—the mass of a substance in a given area. They are also permeable and porous. Porosity refers to the number of spaces between particles in a substance. Porous rocks have tiny spaces, called pores, that allow substances such as oil to collect. Permeability refers to the ease with which substances move through a material.
Oil and gas move through the permeable reservoir rock. Some of it will reach Earth’s surface and seep out. More often, however, it will reach a kind of impermeable rock called cap rock. The cap rock holds the oil in place. Over many thousands or millions of years, the oil slowly builds up, forming a reservoir. These reservoirs are called oil and gas traps.
The tectonic plates can also cause entire reservoirs to move over millions of years. These complex motions make oil and gas reservoirs very difficult to find.