Energy Transfer: Exothermic Processes
Glassblowers all work with torches. A torch is a tool that produces controlled fire at the end of it. The torch produces the extreme temperatures needed to shape and form the particular shapes needed in glassblowing. Torches work because of a chemical reaction.
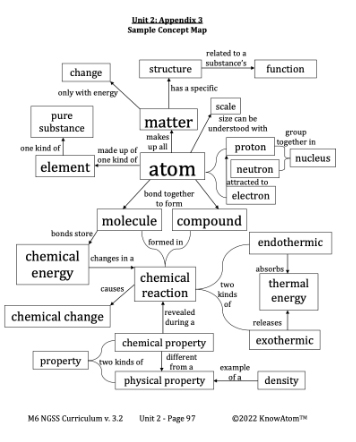
In any chemical reaction, the reactants and products form a system. The environment is everything else, including the air or any substance mixed with the reactants. As the reactants combine and rearrange, energy is exchanged between the system and the environment.
Every chemical reaction needs energy to get started. This initial input of energy is called activation energy. For example, when someone strikes a match to light a candle, they provide the activation energy needed to start a fire, which is a chemical reaction.
Every chemical reaction needs energy to get started. This initial input of energy is called activation energy. For example, when someone strikes a match to light a candle, they provide the activation energy needed to start a fire, which is a chemical reaction.
Once the reaction begins, some reactions absorb more energy from the environment than they release. Others release more energy into the environment than they absorb. Any process in which the system loses heat to the environment is called exothermic. “Exo-” means to give off. Because the energy is released as heat, the environment’s temperature will increase. The environment’s temperature increases because it means that the reaction has released thermal energy into the environment.