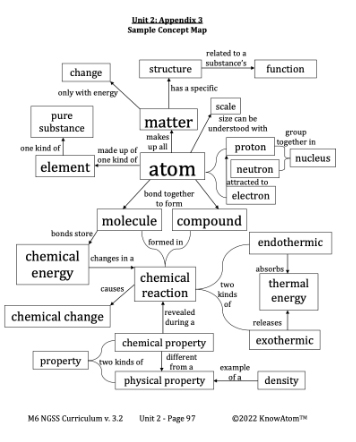
The science background section gives teachers more in-depth information on the phenomena students explore in this unit. Below is an excerpt from this section on atoms.
Kinds of Glass
Kiva Ford became interested in glassblowing because of glass’s unique state of matter. “My experience with glass was that it’s solid,” Kiva said in an interview with the e-commerce website Etsy. “And then when I was introduced to glassblowing, all of a sudden it’s not a solid anymore. It’s a liquid. It almost feels like honey in between your hands.”
Glass is unlike any other kind of substance. It is made primarily from sand, and it can occur naturally or be made by humans. Naturally occurring glass can be made when lightning strikes the sand, during a volcanic eruption, when a meteorite hits sand, or when a comet explodes near the surface.
In all of these cases, extreme temperatures cause the sand to melt. It then cools off very rapidly, a process that results in the unique properties of glass. A property is an observable or measurable characteristic of a substance. Properties include mass, texture, flexibility, color, state of matter, and odor. For example, glass is smooth and transparent. However, there are different kinds of glass depending on how the glass is made. When lightning strikes sand, it forms a glassy tube, often in the shape of a miniature lightning bolt.
In contrast, scientists believe that a meteorite or comet created a kind of glass called Libyan Desert glass because it is found in the Libyan Desert (part of the Sahara Desert). This glass is generally yellow, and it can be translucent or almost opaque. Translucent substances can partly be seen through, while opaque substances block all light. This glass is more than 26 million years old, and people have been using it for millions of years. For example, a large piece of Libyan Desert glass was found in a necklace of Tutankhamen, the Egyptian pharaoh who ruled between 1331-1323 BC.
The Structure of Matter
Glass is a kind of matter, as is sand. Matter is anything that has mass and takes up space. Both sand and glass are kinds of matter because they are both made up of atoms. An atom is the smallest piece of matter that has the properties of an element—substances that are made up entirely of one kind of atom.
The air around you is matter, as are all living things and all of the “stuff” that surrounds you. Energy is not matter because it isn’t made up of atoms. However, energy and matter are constantly interacting. Matter can only change when enough energy is present. To change means to make something different from what it is now.
To understand why a substance has the properties it does, scientists begin with the structure of the atoms that make it up. Structure is the way in which parts are put together to form a whole. The structure of an atom is directly related to the function of matter. Function is the normal action of something or how something works.
Atomic Scale
Atoms are so tiny that we cannot see them without special instruments. Just one grain of sand is made up of many millions of atoms. Because of this, scientists use scale to better understand the size of an atom, its smaller parts, and how it relates to everyday substances. Scale is the size, extent, or importance (magnitude) of something relative to something else. For example, think about all of the atoms that make up a grapefruit. If each atom were the size of a blueberry, the grapefruit would be the size of Earth. There are so many atoms in just one grapefruit that they are impossible to count. Imagine having to fill up the entire planet with blueberries. That’s about how many atoms are in one grapefruit. Atoms themselves are made up of smaller particles, called protons, neutrons, and electrons. These smaller particles are called subatomic particles.
These smaller particles are much smaller than the atom itself. For example, the protons and neutrons group together in the atom’s core, called the nucleus. If you were to open up the blueberry (representing the atom), the nucleus would be too small to see.