In this lesson, students build and test static charge detectors (electroscopes) to observe how materials with high and low static charge exert different levels of electric charge.
In this unit, students focus on the science phenomena of electric and magnetic forces. In this lesson, students build on their knowledge of forces by exploring electric forces. They analyze how materials can become either positively or negatively charged, and then use an electroscope to explore how opposite charges are pulled toward one another and like charges are pushed away from one another. This page showcases all parts of the lesson.
The science background gives teachers more in-depth information about the phenomena students explore in this unit. Below is an excerpt from the background information on static charge.
Although lightning strikes Earth millions of times a day, there are still many unanswered questions, including how it gets started, how it moves, and why it strikes one thing and not the other.
Scientists do know some facts about lightning, including that it is a result of interactions at the atomic scale. Remember that all of matter is made up of atoms, which are themselves made up of smaller particles called subatomic particles.
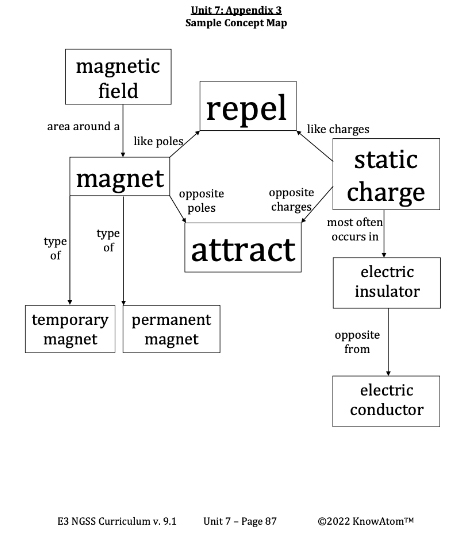
Protons are the positively charged subatomic particles, and they are found in the nucleus along with neutrons, which have no charge. Electrons are the negatively charged subatomic particles of the atom, and they orbit the nucleus because their negative charge is attracted to the positive charge of the protons. The rule is that opposite charges attract and like charges repel. To attract means to pull together. To repel means to push apart.
The number of each subatomic particle a material has determines its electric charge. Electric charge is the property of having an imbalance of positively or negatively charged particles. A negative charge occurs when a material has more electrons than protons. A positive charge occurs when a material has more protons than electrons. The buildup of electric charge in an object is called static charge.
Static charge can happen when two objects rub against each other. Because electrons are attracted to positively charged materials, if a negatively charged material meets a positively charged material, the materials will attract due to their opposite charges. In some materials, electrons move from one object to the other. The material with more electrons than protons now has a negative charge, and the material with fewer electrons has a positive charge.
In this lesson, students build and test static charge detectors (electroscopes) to observe how materials with high and low static charge exert different levels of electric charge.

Prepared hands-on materials, full year grade-specific curriculum, and personalized live professional development designed to support mastery of current state science standards.
Misconception: All metals are attracted to magnets.
Fact: Not all metals are attracted to magnets. Iron, cobalt, and nickel are the three naturally occurring metals that are attracted to magnets.
Misconception: Larger magnets are always stronger than smaller magnets.
Fact: A magnet’s strength depends on the materials that make it up. With two magnets of the same material, the larger magnet will be stronger than the small magnet. But in magnets made up of different materials, the smaller magnet may actually be stronger than the larger magnet.
Attract : to pull together
Electric Conductor : a material that electrons can easily pass through
Electric Insulator : a material that electrons cannot pass through easily repel – to push apart
Static Charge : the buildup of electric charge in an object

What is Lightning?
Scientists know some facts about lightning. Lightning flashes happen because of atoms. Atoms are the tiny parts that make up all matter. The air is made up of atoms. Water is made up of atoms. Grass and trees are made up of atoms.
Every atom has smaller parts. It has protons. Protons have a positive charge (+). They stay in the nucleus. The nucleus is in the center of the atom.
Every atom also has electrons (-). Electrons have a negative charge. Electrons orbit the nucleus.
Opposites Attract
The positive charge of the protons attract negatively charged electrons. To attract means to pull together. Opposite charges attract each other. This is a pulling force.
Like charges repel. To repel means to push apart. Two negative charges push away from each other. Two positive charges also push away from each other. This is a pushing force.
Sometimes the negative electrons separate from the positive protons of an object. This causes objects to have either a positive electric charge or a negative electric charge. The buildup of electric charge on an object is static charge.


Static Electricity
Static charge can happen when two materials rub against each other. In some materials, electrons move from one material to the other. The material with more electrons now has a negative charge. The material with fewer electrons now has a positive charge.
When a child goes down a plastic slide, the child rubs against the slide as they go down it. Electrons move from the child to the slide. A static charge builds up. The slide has more electrons. This gives it a negative charge. The child now has fewer electrons. She has a positive charge. Each of the child’s hairs has the same positive charge.
These positive charges repel each other. Each piece of hair pushes away from every other piece of hair. The hairs try to get as far from each other as possible. This makes the child’s hair stick straight up.
Something similar happens when you take off a hat. Electrons move from your hair to the hat. Your hair is positively charged. This is why it sticks up.
Surfaces that build up static charge are usually insulators. Electrical insulators don’t allow electrons to pass through easily. This is how the electrons can build up in the object. Glass, rubber, plastic, and ceramic are good insulators.
Electrical conductors are materials that electrons can easily pass through. Metals are common conductors. Silver, copper, bronze, and aluminum are all metals. They are good electrical conductors.
In this lesson, students build a model electroscope to measure the electric force produced by the static charge of different materials when those materials are rubbed with wool. Students measure and record the distance between the foil sheets on the electroscope with a ruler and then compare the measurements from each material. Then, students use the data they gathered in their investigation to construct an explanation about the relationship between the amount of static charge a material has and the electric force it produces.
KnowAtom incorporates formative and summative assessments designed to make students thinking visible for deeper student-centered learning.
Standards citation: NGSS Lead States. 2013. Next Generation Science Standards: For States, By States. Washington, DC: The National Academies Press. Neither WestEd nor the lead states and partners that developed the Next Generation Science Standards were involved in the production of this product, and do not endorse it.
